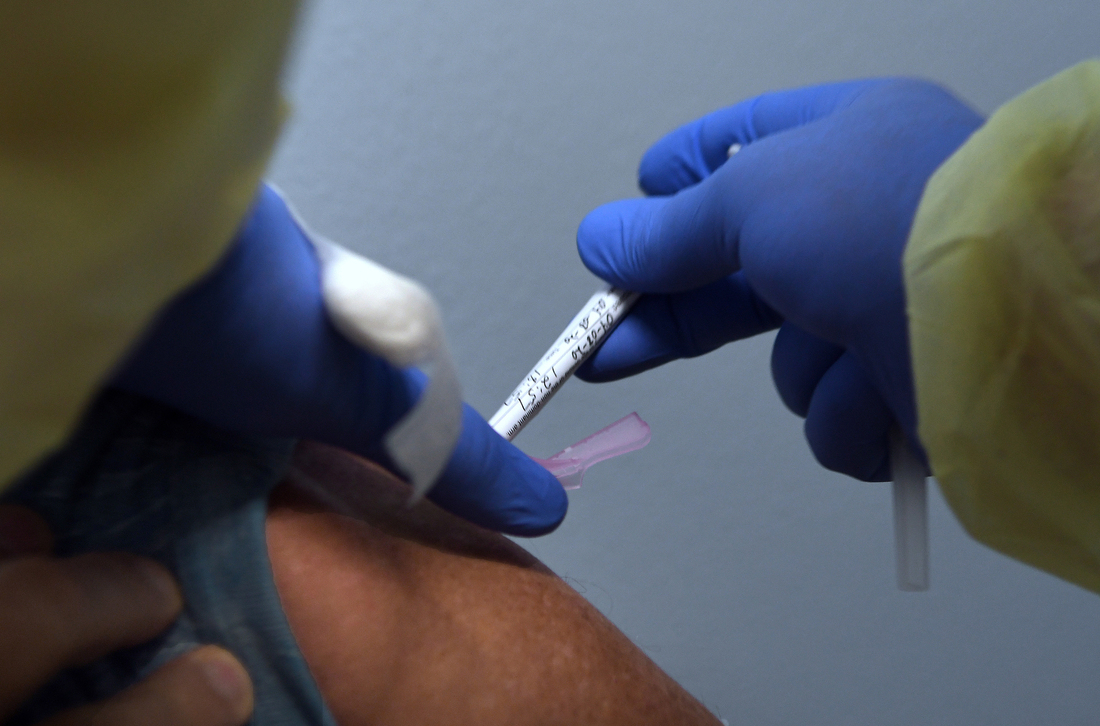
Tony Potts, a 69-year-old retiree living in Ormond Beach, Fla., receives his first injection earlier this year as a participant in a Phase 3 clinical trial of Moderna’s COVID-19 candidate vaccine.
NurPhoto via Getty Images
hide caption
toggle caption
NurPhoto via Getty Images
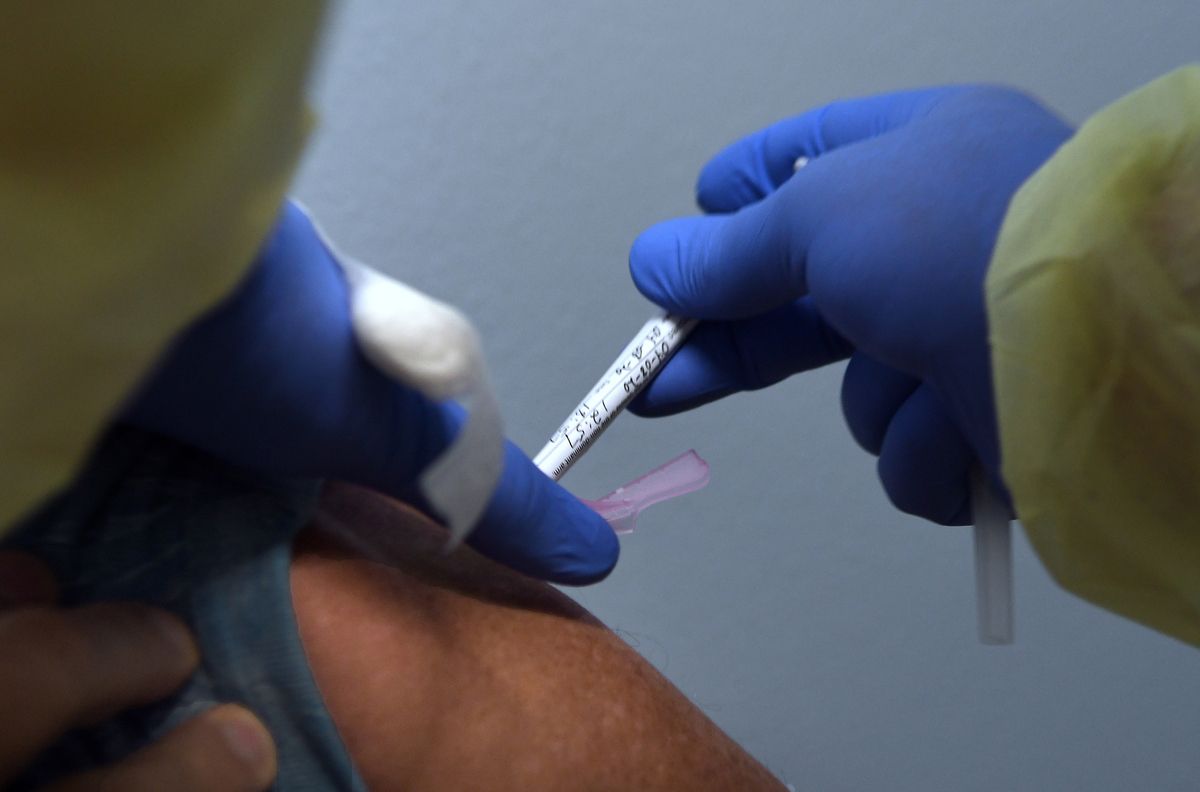
Tony Potts, a 69-year-old retiree living in Ormond Beach, Fla., receives his first injection earlier this year as a participant in a Phase 3 clinical trial of Moderna’s COVID-19 candidate vaccine.
NurPhoto via Getty Images
States should be working toward being ready to give out COVID-19 vaccines by Nov. 15, according to a target date made public by the Centers for Disease Control and Prevention on Friday.
That’s an aspirational date so far — there is still no vaccine approved for use, and there may not be one until later this year or beyond. But, in preparation for that day, the CDC’s Advisory Committee on Immunization Practices, a group composed mainly of doctors and public health experts outside of CDC, met virtually Friday and debated how best to distribute such a vaccine when it becomes available, weighing who would be in line to get it first.
Once a COVID-19 vaccine is authorized by the Food and Drug Administration, ACIP will make recommendations on how the vaccine should be used. Its guidelines will trigger the start of the vaccine distribution process, according to Paul Mango, a leading official at the Department of Health and Human Services.
“[The government] has plans to distribute vaccines within 24 hours after the ACIP gives its final approval,” Mango told reporters in a press call on Oct. 23.
At ACIP’s meeting Friday, members reviewed what’s known about the candidate vaccines now in clinical trials, and laid groundwork so they can make rapid decisions once a vaccine is authorized.


In a presentation to the committee, Dr. Mary Chamberland, representing the CDC, said ACIP has agreed to follow the principles of maximizing benefits and minimizing harms, promoting justice and mitigating health inequities in determining early allocation groups.
ACIP discussed four overlapping groups who are at high risk of getting COVID-19 that might be prioritized in getting the vaccine. The schedule of which groups get the vaccine when will depend on characteristics of the vaccine and quantities available, the advisers said.
Still, a consensus has formed that health care personnel should be first in line to get the vaccine, given their high risk of exposure. Health care workers are defined by ACIP as “paid and unpaid persons serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials.” This population is estimated at 21 million.
A second group outlined by ACIP would be much larger — 87 million. This would include workers in other essential areas, defined as persons who “conduct operations vital for continuing critical infrastructure, such as food, agriculture, transportation, education and law enforcement.”
A third group, of around 100 million people, would be those who have health conditions that put people at higher risk of serious COVID-19 disease. These are adults who have one or more high-risk medical conditions, such as obesity, diabetes, and cardiovascular disease.
A fourth group that would be considered for priority vaccination are adults 65 years and older (53 million people) who have no other health conditions, but who are at risk for serious disease based on their age. This includes adults living at home and adults living in long-term care facilities (3 million), the CDC says.
ACIP’s goals are to use a vaccine to reduce transmission, illness and deaths from COVID-19, and there are different strategies that are being considered to achieve that.
To that end, a modeling study presented by CDC epidemiologist Matthew Biggerstaff showed that vaccinating all adults 65 years and older first (after health care workers) might have the greatest effect on reducing the overall number of deaths in the U.S.
Biggerstaff’s presentation also suggested that vaccinating high risk adults of any age who have underlying medical conditions — such as lung disease, heart disease, diabetes, kidney disease or obesity — might have the greatest effect on reducing the number of infections, and thus limit the spread of the coronavirus.
Biggerstaff cautioned that these are theoretical scenarios and that the actual outcomes may differ, depending on the characteristics of whichever vaccines become available.

CDC medical officer Dr. Janell Routh told the committee that states and territories are working toward a “readiness date” of Nov. 15 for vaccine distribution. “We want to ensure before product is rolled out that everything is in place to accept and administer vaccine,” she said.
There are 64 jurisdictions, including states, territories and some large cities, that have submitted preliminary plans to the CDC for distributing the vaccines. The CDC provided feedback on the plans this week, and expects states to be enrolling providers, setting up data systems to track who’s getting vaccines, and working with community leaders, so states are ready to give out vaccines as soon as one is authorized by the FDA.
Plans are also being made to augment state capacity to distribute vaccine in long-term care facilities “through federal pharmacy partnerships,” according to Routh. A revised “playbook” for states to use in developing plans was released Friday and notes these partners might include national chains, large regional chains or networks of independent pharmacies. Routh said that 55% of eligible U.S. pharmacies have already signed up to give out COVID-19 shots.
Once a vaccine is approved, the CDC is planning a national “Vaccinate With Confidence” campaign with extensive outreach to health care providers and various communities to encourage people to get it. Dr. Amanda Cohn, the CDC’s acting chief medical officer of vaccine policy, said the public health campaign will be based on “evidence-based content to amplify messages that enable an individual to make the decision to vaccinate.”
The panel also heard data from two manufacturers of candidate vaccines, Novavax and Janssen, about progress with their studies, which are still enrolling volunteers. It’s not certain if these two vaccines would be among the first to be released.
It’s up to the FDA to approve or give emergency authorization to any vaccine. There are currently four candidate vaccines in the final phase of clinical study in the U.S. None of the companies so far has applied for authorization or approval from the FDA.
For the latest news and updates, follow us and if you like our efforts, consider sharing this story with your friends, this will encourage us to bring more exciting updates for you.
